
|
Skin Pharmacies
.ca
Written by Dermatologists for Pharmacists.
|

|
| Skin Pharmacies | Free Subscription | About Us | Dermatology Glossary and Images | Skin Care Network | Skin Pharmacies INDEX |
A Review of Therapeutic Options for Genital Warts
M. Gooderham, MSc, MD, FRCPC
Peterborough, ON, Canada
Introduction
Condylomata acuminata (genital or venereal warts) pose a significant health concern, especially amongst young adults. Considered to be one of the most common forms of sexually transmitted infections (STIs), external genital warts (EGWs) are caused by infection with the human papillomavirus (HPV), the same virus that causes the majority of cervical cancers. Relatively recent therapeutic advances include a topical immunomodulatory agent and a prophylactic vaccine, which have significantly broadened the options for management. Herein, a review of conventional and newer therapies will be discussed.
HPV Facts
Over 100 HPV types have been described, 40 of which infect the anogenital tract; the most common of these are HPV types 6, 11, 16, and 18.1
- HPV 6 and 11 are low risk for causing cancer, but they cause 90% of genital warts.
- HPV 16 and 18 are considered to be high risk types and contribute to 70% of cervical cancers.
HPV Pathogenesis
The principal route of genital infection is through sexual contact. The virus is believed to enter through micro-abrasions in the epithelium. Transmission to newborns by way of passage through the infected birth canal can also occur.2 The infection rate between sexual partners is approximately 60%.3
Risk Factors
It is estimated that 550,000 Canadians are infected with HPV annually.4 The rate of infection appears to accelerate following the onset of sexual activity and decrease with increasing age. Prevalence is highest amongst individuals under 25 years of age. The risk level is based on a combination of factors including age, lifestyle, immunocompetency, and other variables.
- Becoming sexually active at an early age
- Previous infection with another form of STI
- The lifetime number of sexual partners
- Engaging in unprotected sex with multiple partners, especially those with known histories
- The sexual promiscuity of partners
- Immune status
- Proper condom use may reduce the risk of transmission, but because HPV can be present anywhere along the anogenital tract only partial protection is provided.
- Male circumcision may reduce the incidence of HPV infection according to a recent study by Tobian, et al.5
Diagnostic Features
Prior to making a confirmed diagnosis, it is necessary for clinicians to obtain a medical and sexual history from patients, if not already known. Examination of the pelvic region, entire genital tract, and the thighs, as well as mouth and throat, may reveal nodules indicative of, but not limited to, infection with HPV. Screening for other STIs may also be considered, especially in high-risk individuals.
- In most cases, direct visual inspection can identify the growths on the genital mucosa.
- Anogenital warts are generally asymptomatic, but they can cause pruritus, bleeding, or mild burning. They present as:
- lesions that appear primarily on surface areas of the vulva, penis, and perianal skin.
- small, discrete, sessile, flat or raised papules or nodules.
- large exophytic masses.
- a singular papule or multiple verrucous clusters.
- lesions that range in colour from whitish, pink, flesh-coloured to reddish brown.
- having a multifocal distribution that generally correlates with regions sustaining the greatest degree of friction during sexual activity.
- For hard-to-see warts, a 3%-5% acetic acid solution (i.e., white vinegar) can be applied to the suspect lesion (Figure 1). After a few minutes, the condylomata should appear as whitened patches on the mucosa. Positive changes are not diagnostic for HPV, as these results can also be produced by lichen planus, yeast infections, and other skin disorders.
- A biopsy may be considered if:6
- diagnostic uncertainty exists.
- lesions are unresponsive to convention treatments.
- lesions worsen during therapy.
- the patient is immunodeficient.
- the warts are pigmented, indurated, fixed, bleeding, or ulcerated.
- The lesion prevalence in women may be attributable to larger surface areas of mucosal skin.
- A Pap test can help to establish the presence of a cervical lesion.7
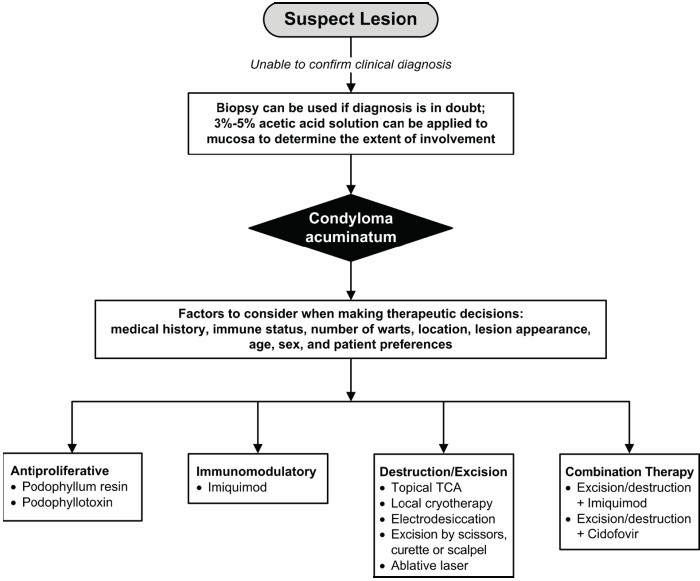
Figure 1: Algorithm for treatment of suspect lesions8
Treatment
The primary treatment objectives are to eliminate visible warts and limit the psychological distress caused by EGWs. In about 10%-30% of patients, EGWs are usually self-limited in immunocompetent individuals and typically resolve within 12-24 months if left untreated; however, lesions may also remain unchanged, or proliferate in size and number.6,7
- The spectrum of available treatments includes self-applied and provider-administered therapies.
- The most widely used treatment modalities can be broadly categorized as antiproliferative, destruction/excision,immunodulatory, and combination therapies (Table 1).
- The majority of therapeutic options provide symptomatic relief rather than treat the disease itself. The one exceptionis a topical immune response modifier (i.e., imiquimod), which exerts a field effect that can target both clinical andsubclinical manifestations of HPV.
- Therapeutic decisions should be guided by wart type, location, number, sex, patient preferences, physician experience,and unique circumstances (e.g., young age, immunosuppression, pregnancy).
- Given the wide range of patient and treatment variables, there is a lack of conclusive evidence confirming the superiorityof any one modality, or combination thereof, over another.
- The majority of patients require a course of therapy rather than a single treatment.6
- It is important to iterate to patients that available treatments can induce wart-free periods, but none provide completeclearance of HPV infections.
| Method | Treatment | Comments |
| Antiproliferative Therapies |
Podophyllum resin 10%-25% |
|
|
Podophyllotoxin 0.5% solution or gel |
|
|
|
Immunomodulatory Therapy |
Imiquimod 5% cream |
|
|
Destruction/Excision Therapies All options have the potential to cause scarring |
Topical trichloracetic acid 85% (TCA) |
|
| Local cryotherapy |
|
|
| Electrodesiccation |
|
|
|
Excision by scissors, curette, or scalpel |
|
|
| Ablative laser |
|
|
|
Combination
Therapy
Combination therapy can provide a better result over monotherapy |
Excision/destruction + imiquimod |
|
|
Excision/destruction + cidofovir |
|
Table 1: Treatment options for genital warts7,10 |
The therapeutic horizon may include a topical formulation whose active constituent is a defined mixture of catechins extracted from green tea with demonstrated efficacy and safety for EGWs.11 This herbal prescription drug gained US FDA approval in 2006 for the treatment of external genital and perianal warts caused by certain strains of HPV.
A Prophylactic Approach
In 2006, Health Canada approved a quadrivalent HPV vaccine that acts against HPV types 6, 11, 16 and 18.- It is indicated for use in females 9-26 years of age and is given as a 0.5ml injection intramuscularly in 3 doses at 0, 2, and 6 months.
- The quadrivalent vaccine is 97% effective in preventing vaginal and vulval intraepithelial neoplasia, and is 99% effective in preventing genital warts caused by HPV types 6 and 11.1
- There is no evidence suggesting that therapeutic benefits may be derived from the immunization vaccine if patients are already infected with vaccine HPV types.
- Recent studies suggest that the quadrivalent vaccine may also provide cross-protection against HPV strains that are not contained in the vaccine, but are closely related. However, durability of immunity and the significance of these findings remain to be established.12,13
- Studies investigating this vaccine in males are underway.
Conclusion
The increasing incidence of HPV infections is of mounting concern and the most prevalent clinical manifestation of this communicable disease is genital warts. Although disease morbidity can be mild, the emotional distress of having genital warts can result in severe psychological impacts, hence, successful management is essential.
- For the initial treatment of genital warts, many patients prefer self-applied therapies.
- Because monotherapy is often insufficient, combination therapy may be more advantageous.
- Throughout the course of treatment, patients must be monitored for response rate and side-effects.
- Patients exhibiting an inadequate response will necessitate a transition to other therapies or modification of the existing approach.
- According to the Canadian Consensus Guidelines on HPV,7 due to its favourable efficacy, safety, and tolerability profiles, as well as lowest recurrence rate, imiquimod represents an effective strategy for the management of genital warts, and should be considered prior to initiating more invasive strategies, such as destructive/excision or laser therapies.
References
- Dobson S, et al. Canada Communicable Disease Report 33(ACS-2):1-31 (2007 Feb 15).
- Kaye JN, et al. J Gen Virol 77 (Pt 6):1139-43 (1996 Jun).
- Palefsky JM. 15(3):439-47 (1997 May-Jun).
- Money DM, et al. J Obstet Gynaecol Can 29(8 Suppl 3):S3-6 (2007 Aug).
- Aaron AR, et al. N Engl J Med 360(13):1298-309 (2009 Mar 26).
- Centers for Disease Control and Prevention. Genital warts treatment guidelines 2006. Available at: http://www.cdc.gov/std/treatment/2006/genital-warts.htm. Accessed March 17, 2009.
- Roy M, et al. J Obstet Gynaecol Can 29(8 Suppl 3):S37-41 (2007 Aug).
- Varela A, et al. Skin Therapy Lett-FP US Ed 1(2): 1-3 (2006).
- Carrasco D, et al. J Am Acad Dermatol 47(4 Suppl):S212-6 (2002 Oct).
- Bourcier M, et al. Skin Therapy Lett-FP Ed 3(2): 1-3 (2007 Jun).
- Stockfleth E, et al. Br J Dermatol 158(6):1329-38 (2008 Jun).
- Brown DR, et al. J Infect Dis 199(7):926-35 (2009 Apr 1).
- Wheeler CM, et al. J Infect Dis 199(7):936-44 (2009 Apr 1).
Other articles from this issue: